Abstract
Background: Acute myeloid leukemia (AML) is an aggressive hematologic malignancy that carries a poor prognosis in adults despite significant contributions to understanding disease biology and therapeutic advances in recent years. Hypomethylating agents (HMA) in combination with the BCL-2 antagonist, venetoclax (VEN), has become standard of care in the upfront setting for those not eligible to receive intensive chemotherapy and is frequently used in the treatment of relapsed or refractory (R/R) disease. The aim of this study was to characterize response and survival of patients with AML receiving HMA/VEN in both the upfront and R/R settings with respect to disease mutation profiling.
Patients & Methods: We analyzed 103 patients (53 upfront and 50 R/R) with AML treated with HMA/VEN from January 2016 to December 2021 at VCU Massey Cancer Center. Patient and disease characteristics, cytogenetic and molecular profiling, therapies, response, and dates of death/last contact were obtained through retrospective chart review. Overall survival (OS) was determined by the Kaplan-Meier method with significance determined by the Mantel-Cox test. The event for calculating the OS was the date of death. Patients were otherwise censored at the date of last contact.
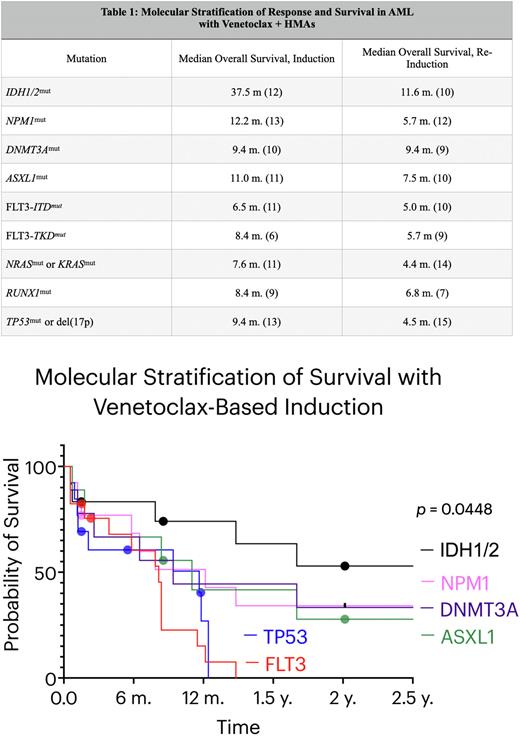
Results: Fifty-three patients were treated with HMA/VEN in the upfront setting. Patients with epigenetic regulator mutations at time of diagnosis had the following OS: the IDH1/2mut cohort demonstrated an OS of 37.5 months, NPM1mut 12.2 months, DNMT3Amut 9.4 months, and ASXL1mut 11.0 months. In the signaling pathway cohort, the OS of FLT3-ITDmut was 6.5 months, FLT3-TKDmut 8.4 months, and 7.6 months for NRASmut or KRASmut. Lastly, for the transcription factor cohort, the OS of RUNX1mut was 8.4 months and 9.4 months for TP53mut or del(17p). The Mantel-Cox test demonstrated a statistically significant difference in OS between those with IDH1/2mut in the upfront setting and the remaining cohorts (p = 0.0448). We then analyzed 50 patients that received HMA/VEN in any line in the R/R setting. The OS of IDH1/2mut was 11.6 months, NPM1mut 5.7 months, DNMT3Amut 9.4 months, and ASXL1mut 7.5 months. FLT3-ITDmut and FLT3-TKDmut had an OS of 5.0 and 5.7 months, respectively, and the RASmut cohort was 4.4 months. Finally, relapsed/refractory RUNX1mut was 6.8 months and TP53mut was 4.6 months (Table 1).
Conclusions: When stratified by molecular profiling alone (without respect to cytogenetics), treatment of IDH1/2mut disease with HMA/VEN appears to offer a significantly increased survival benefit compared to the remaining molecular cohorts analyzed. Ongoing work will determine the event free survival (EFS) and any contribution of IDH1/2 targeted therapies in subsequent lines that may contribute to this finding. Interestingly, the increased survival achieved with IDH1/2mut disease was also observed in the R/R setting. HMA/VEN may offer improved OS for TP53mut or del(17p) in the upfront setting compared to historical controls, yet prognosis for these patients in the R/R setting remains poor. Our data also highlights the relative refractoriness of FLT3mut disease with HMA/VEN, suggesting need for other therapeutic approaches, such as those incorporating FLT3 inhibition.
Disclosures
Maher:BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal